The company said in a statement that the trial would be carried out at the University of the Sunshine Coast's Sippy Downs clinical research set-up.
Vaccines delivered by using this technology are being investigated and are only available for investigational uses.

A Vaxxas team member working with the HD-MAP technology in the company's labs.
The statement said if the trial was a success, then Phase II and II studies would follow. If these also came up positive, then a patch could be available as early as 2025.
|
|
"The vaccine candidate is a second-generation version of the spike protein used in the major US-approved COVID-19 vaccines, and has been modified for stability and immunogenic response, giving potential coverage of all known SARS-CoV-2 variants," the statement said.
"Results from pre-clinical animal studies completed in July of this year support the potential efficacy of the COVID-19 vaccine patch against all current variants of concern."
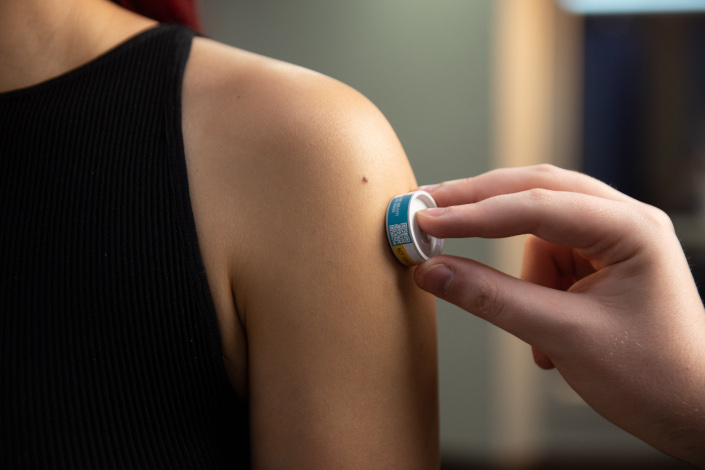
A patch being applied.
Vaxxas chief executive David Hoey said: “Attaining this clinical milestone and building upon compelling pre-clinical data, we are excited by the rapid progress of our needle-free COVID-19 vaccine candidate.
“Vaxxas’ HD-MAP technology can potentially enable cost-effective distribution without the need for extensive refrigeration, and our vaccination patch offers the potential for self-administration. This may enable an accelerated response in a pandemic situation and broader population coverage.”
The trial will test safety, tolerability, and immunogenicity of the COVID-19 vaccine candidate in 44 healthy adults, aged 18 – 50 years inclusive, who have had three doses of a COVID-19 vaccine, with the last dose received at least four months before the study.
The trial is also designed to gather signals related to antibody and T-cell responses to dosing with the patch-delivered vaccine candidate.
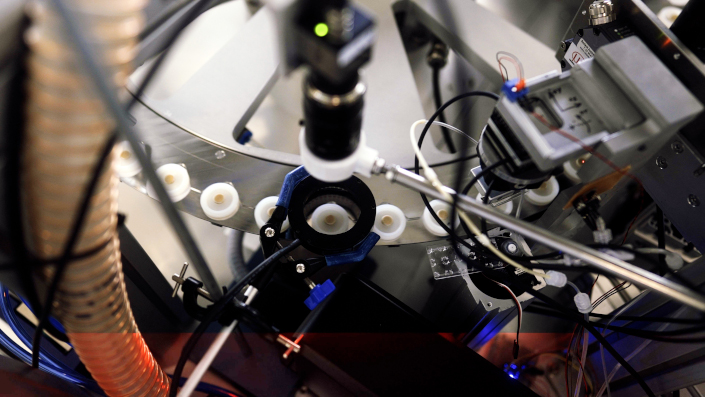
Printing the HD-MAP technology on a patch.
“At a time when the world is facing more emerging variants of the COVID-19 virus, it’s especially important to continue to build out our arsenal of tools to prevent infection and serious disease,” said Jason McLellan, a professor of molecular biosciences and Welch Chair in Chemistry at The University of Texas in Austin.
“Clinical testing of this patch-based vaccine that uses UTA’s vaccine candidate represents a significant step towards equipping the globe for new phases of the fight against COVID-19.”
Scientific publications Science Advances and Vaccine have published research undertaken by The University of Queensland and collaborators showing the UTA vaccine candidate resulted in enhanced virus-neutralising antibody and T-cell responses against all known variants of concern, including alpha, beta, gamma, delta, and omicron, when compared to needle and syringe vaccination with the same vaccine.
“We are pleased to see our COVID-19 patch transition from the lab to Vaxxas for Phase I clinical trials,” Dr David Muller of The University of Queensland said.
“Our work demonstrated that the COVID-19 vaccine patch, when tested in mice, produces potent immune responses against every SARS-CoV-2 variant we tested, including delta and omicron in pre-clinical models. If these results translate to humans, this patch could be a great tool in the fight against COVID-19.”
Vaxxas is a private company focused on improving performance of existing and next-generation vaccines using its HD-MAP technology.
All photos courtesy Vaxxas.