In a statement, the university said the researchers used nickel, iron and cobalt to create a series of porous, metal-organic framework nanocrystals, which presented a unique atomic structure and many exposed surfaces throughout the material.
The team was led by FH Loxton Research Fellow Dr Shenlong Zhao from the School of Chemical and Biomolecular Engineering. The study was funded by an FH Loxton Research Fellowship.
A paper on the team's work has been published in Nature Energy. The other authors of the paper are Chunhui Tan, Chun-Ting He, Pengfei An, Feng Xie, Shuai Jiang, Yanfei Zhu, Kuang-Hsu Wu, Binwei Zhang, Haijing Li, Jing Zhang, Yuan Chen, Shaoqin Liu, Juncai Dong and Zhiyong Tang.
|
|
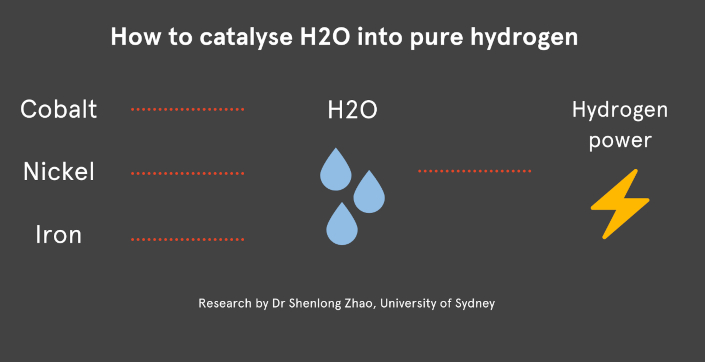
Precious metals like like platinum or ruthenium were normally used as catalysts, contributing to the exorbitant cost.
Additionally, the huge amount of energy needed is a drawback and the required catalyst materials can break down well before the necessary change occurs.
The university said, when a hydrogen fuel cell created electricity, water and heat were the only by-products, meaning that the use of hydrogen would help reduce carbon emissions.
It cited a Bloomberg report as saying that South Korea, Japan and Germany were the current leaders in hydrogen energy development.
"Our team has developed an exciting new catalyst based on iron, cobalt and nickel that can be used to create pure," said Professor Dianne Wiley, Head of the School of Chemical and Biomolecular Engineering.
"We are on the cusp of the hydrogen age: the adoption of large-scale technology is finally within striking distance. Targeted investment and research in this area would usher in a new age of truly transformative renewable energy," lead author Dr Zhao said.
"Improvements in energy conversion and storage are absolutely essential for a successful and sustainable energy economy. Because energy from solar and wind sources is intermittent, our research sought to discover an efficient way of storing renewable-sourced power."
Dr Zhao, who was the first researcher to synthesise ultrathin metal-organic framework nanosheets for water splitting, added: "Just recently we witnessed a worldwide commitment to a clean energy future. What we now require is significant investment from industry and government to wholly develop hydrogen technologies."
He believes research like this could be used to develop long-range hydrogen-powered aircraft and fuel cells for industrial uses.
His colleague and recipient of the 2020 Prime Minister's Prize for Innovation, Professor Thomas Maschmeyer, said Australia was well-placed to take advantage of the technology.
"Australia is extremely well-placed to advance green hydrogen technology, both as manufacturer and consumer. Hydrogen can be used for energy storage as well as an agent replacing gas, oil and coal," said Professor Maschmeyer who is from Sydney Nano and the School of Chemistry.
"Not only are we the world's largest iron-ore producer and a leading supplier of nickel and cobalt, but our abundance of sunshine and wind means that hydrogen could transform our domestic energy system as well as create many opportunities in sustainable manufacturing."
The NSW Government recently announced a $32 billion renewable energy plan.